<Cancer Genome Analysis> Proposal of the effect judgment method that uses genome analysis to determine the therapeutic effect of solid cancer
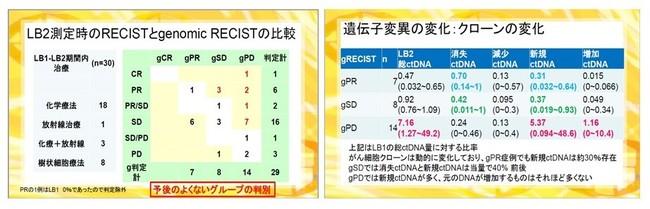
In this announcement, based on the case data analysis of the Tokyo Midtown Advanced Medical Research Institute, a liquid biopsy test * 1 "Genomic Recist) using a liquid biopsy test * 1 result is devised, and clinically in the clinical clinical. The usefulness of is suggested. * 1 Inspection that analyzes cancer genetic information from body fluids such as blood without using a tumor tissue. ■ What is "Genomic Recist" advocated by Dr. Taguchi? RECIST guidelines, which are determined for therapeutic effects of solid cancer, are evaluated in four steps based on the size (amount) of the tumor (quantity) by image diagnosis such as CT and PET, and this standard is adopted in Japan and overseas. * 2 However, the research results of the Tokyo Midtown Advanced Medical Research Institute have shown that the size of the tumor and the detection of cancer gene mutation are not always proportional. Dr. Taguchi devised "Genomic Recist", which determines the effect of cancer treatment based on genome analysis results, and performs liquid biopsy analysis as a monitoring of cancer treatment. It shows the importance and the definition of Neo Antigen identification. * 2 From the National Cancer Research Center Cancer Information Service https://ganjoho.jp/public/qa_links/dictionary/dic01/modal/recist.html
(From Dr. Taguchi's presentation material)
See below for detailed research announcements on Dr. Taguchi's "Liquid Biopsie Analysis Eating Neo Antigen's identification and Dendrigic Cancer Immunotherapy Initiatives".>>> News Release "Neo Antigen identification using liquid biopsy and application to immune cell therapy" (PDF: 1,850KB) https://www.amcare.co.jp/news/uploads/pr_release_sentan_amc20706.pdf [Doctor introduction]
Junichi Taguchi Doctor Junichi Taguchi, Director of Tokyo Midtown Advanced Medical Research Institute, Tokyo Midtown Clinic Nihonbashimuro Mitsui Tower Midtown Clinic General Director of General Director of General Director of General Director of the University of Tokyo. 1993 He studied at Washington State University. After working as an assistant to the University of Tokyo Hospital, a former shrine inheritance, a samurai physician, and an associate professor at the Tokai University School of Medicine, Hachioji Hospital Cardiovascular Medicine. May 2020 Nihonbashi Muromachi Mitsui Tower He became the director of Midtown Clinic.
