
Do you know what an air battery is? Energy thinking from "quantity" (Mayu Takekoshi)
I wish the battery lasted longer! Have you ever thought that while using a smartphone or computer? Keep in touch with your smartphone, read this blog, work on your computer, make video calls with your family, etc. In this day and age, we rely on battery devices for many things. It may be sudden, but today I would like to talk about such batteries by science communicator Takekoshi, who has recently become addicted to batteries.
What types of batteries do you have around you? Dry cell batteries, button batteries, smartphone batteries, and so many more just by looking at them.
The more I learned about it, the more I fell in love with batteries. Really deep! There are still many things about batteries that cannot be fully explained! So please stay with us for a while.
Do you know a battery called an "air battery"? As the name suggests, it is a battery that uses air (oxygen). Due to its light weight and large amount of energy that can be stored, it is also called the “ultimate secondary battery”. how is it? You're all curious, aren't you?
An air battery is a secondary battery, a type of battery that can be recharged and used repeatedly. Lithium-ion batteries are also used in smartphones and computers that are familiar to everyone.
The air battery is a secondary battery that is currently under development. There are various types of next-generation batteries, depending on the type of electrode, etc., but the air battery is regarded as the "ultimate secondary battery" because of the high expectations.
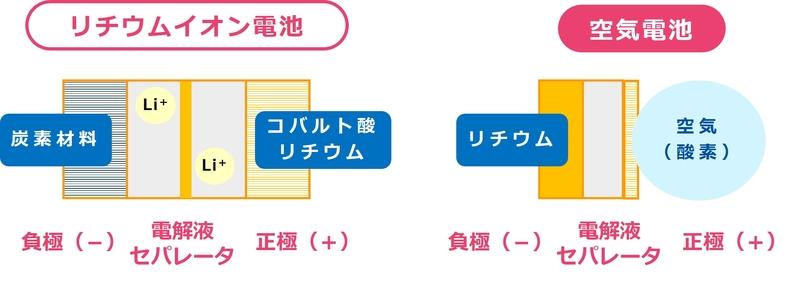
So what is the ultimate? Let's start with the battery materials. A battery is mainly made up of a positive electrode, a negative electrode, an electrolyte (which conducts electricity between the positive electrode and the negative electrode), and a separator (which separates the positive electrode from the negative electrode so that nothing other than electricity can flow). The type of battery changes depending on what materials are used for the parts.
For example, lithium-ion batteries, which are familiar to us, mainly use lithium cobaltate for the positive electrode and carbon material for the negative electrode. Both lithium and carbon are lighter than conventional battery materials, which is one of the reasons why mobile devices have come to be widely used.
As for the air battery, the negative electrode is lithium and the positive electrode is air (oxygen inside)!
In theory, the weight of the battery itself can be reduced by about half because air is taken in from the outside. No matter how light a material is used for the positive electrode, it will still be heavier than nothing (air).
*Actually, the positive electrode is equipped with a layer (porous carbon material) to take in oxygen.
Here, the lightness of the battery leads to the high "energy density". Next-generation batteries are expected to have high energy density, that is, batteries that are light but can store and extract large amounts of energy. Air batteries are theoretically considered to have the highest energy density, which is why they are called "ultimate secondary batteries."
To explore the cutting edge of air batteries, we visited the National Institute for Materials Science (NIMS) Research Center for Energy and Environmental Materials (GREEN). At GREEN, we aim to find fundamental solutions to global environmental problems, and are conducting research to solve energy problems in particular from the perspective of nanotechnology. It is also characterized by an approach from three points of experimentation, measurement, and calculation, which is not just making materials and conducting experiments, but also measuring what is produced and making predictions based on theories based on the results.
NIMS Fellow Professor Kohei Uozaki talks about his thoughts on battery research. “Lithium-ion batteries, which are currently in widespread use, are of a high standard, and next-generation batteries must surpass even that level. I believe that proper research is necessary in order to create batteries that can be used practically. "
Sure, even better than what's in use today! …That hurdle seems high. Air batteries are expected to be the key to overcoming that barrier. How advanced is the research? Let's look at it from a performance standpoint.
In addition to the "energy density" mentioned earlier, "power density" is also important for battery performance. Energy density is how much energy can be stored. Power density is the amount of electricity that can be discharged instantaneously. It works like the accelerator of a car.
For the energy density of the air battery, we have achieved the target value of 500Wh/kg. 500Wh/kg is a performance that exceeds the limit of lithium-ion batteries currently in use, and is required as the performance of next-generation batteries that will be installed in next-generation electric vehicles.
Actually, we have achieved the target value of 50W/kg for power density, but it is lower than other next-generation batteries. Therefore, even if the power density is low, it is expected to be applied as a battery that is slowly charged and used, rather than being used like an electric vehicle battery.
For example, application to a flying mobile phone base station (HAPS) is currently being considered as an application. By floating the base station in the stratosphere nearly 20 km above the ground, it is expected to provide a stable Internet environment even in countries and regions where communication networks are not well established. HAPS is required to automatically fly for several months by storing electricity in a secondary battery while generating electricity from solar cells during the day and using the stored electricity at night. Air batteries, which are light and have high energy density, are said to be perfect for this base station.
Now that we've gotten a little more familiar with the materials and performance of air batteries, let's go see how air batteries are actually made!
Although it is called an air battery, ordinary air is actually a big enemy! ! Ordinary air contains water as humidity. This moisture degrades the lithium and electrolyte used as materials in the air battery. Therefore, when actually creating an air battery, we enter an extremely dry room called a super dry room. (It seems that the skin will become crispy if you stay for a long time ...)
When I went inside, there were various devices. It seems that it is possible to perform everything from battery assembly to measurement in the dry room.
We actually had them assemble a "button battery" type air battery! Check out this video! (about two and a half minutes)
And you can actually turn on electricity by connecting wires like this! I can't tell you in the picture, but of course you can charge the battery and use it again and again when the electricity goes out.
At the laboratory, by examining thousands and tens of thousands of types of the black circle "positive electrode" that appeared in the video and the transparent "electrolyte" that was in the bottle, We continue to search for air batteries. Making materials, assembling a battery, measuring it, and so on... through a tremendous amount of work, research will lead to future batteries.
The air battery is said to be the ultimate battery due to its overwhelmingly high energy density, but it seems that there are still problems.
One of the challenges is the number of cycles. How many times can it be used repeatedly? Even if a battery can store a lot of energy, it cannot be called a secondary battery if it can be used only once, and it seems difficult to actually use it.
Actually, the more you try to increase the energy density, the more difficult it becomes to earn cycles. What is important here is the "reaction of the electrolyte". The major difference from the currently used lithium-ion batteries is whether or not the electrolyte reacts (changes).
In a lithium-ion battery, lithium ions (Li+ in Fig. 1) simply move back and forth between the positive and negative electrodes, but in many batteries, including air batteries, the electrodes are separated by chemical reactions. and the electrolyte itself will change. If 1% of the electrolyte reacts and decomposes each time it is charged or discharged, the electrolyte will stop functioning after 100 repeated uses. There is no problem if there is a lot of electrolyte, but if we are aiming for a practical level, we need to reduce the amount of electrolyte as much as possible. However, if you reduce it too much, even a few percent loss will render the battery non-functional.
Aiming for the ultimate battery, energy density and cycle number are competing.
Professor Shoichi Matsuda, who is doing research at GREEN, is searching for materials by “automation” in order to try more types of electrolytes. By automating everything from making the electrolyte material to measuring it, we were able to search for materials 100 times more efficiently than by doing it manually.
At GREEN, researchers in materials development, measurement, and calculation work together to make sure that the electrolyte does not decompose as much as possible. New electrolytes are being developed.
So far, we have talked about the advantages and goals of air batteries. Finally, we once again asked Dr. Kohei Uozaki about his thoughts on battery development.
――Batteries are closely related to energy issues, but my thoughts on energy are: “Energy needs volume. If you try to compete with the high energy density that is generated when you burn fossil fuels that you use in your daily life, it will suddenly become difficult.”
In order for humans to be active, it is necessary to provide energy through food, and we also receive electricity from power plants and use it in our daily lives. We may have to deal with more energy than we think and have to cover it.
--What role will next-generation batteries play in the future society? If we can store more electricity, we will be able to increase the proportion of natural energy.As I have said, if next-generation batteries such as air batteries are realized, I think that society will change dramatically. "
Energy is indispensable for us to live richly. Now that there are calls for energy depletion and a need for conversion, this battery technology may be the key to that conversion. Among them, it was a story of a researcher who is particular about whether it is the "quantity" that can actually be used. It may be a tough path to take on the cutting edge that no one has ever walked, but I believe that it will change the future for the better, and I would like to pay attention to future research!
[Acknowledgments]
In writing this article, Dr. Kohei Uozaki, Dr. Shoichi Matsuda, and other researchers who cooperated in the interviews, and NIMS for providing the opportunity to interview and tour the facilities, and to guide us every corner. I would like to take this opportunity to express my sincere gratitude to everyone in the public relations department.
Written by Mayu Takekoshi (Science Communicator, National Museum of Emerging Science and Innovation)While pursuing the world in a flask at university, I wondered what research was, and came to Miraikan. As I talk to many people every day, I am struck by their different ways of thinking. I'm looking for ways to connect each person's thoughts to making the future a little better. The pentagons and hexagons are the shapes that I can't help but react to.